Answer:
(a) 5.3 grams
(b) 0.2 years
Explanation:
(a) Step 1: Since we're already given A0, we simply plug in 0.5 for t to find A(0.5), aka the amount of Thorium-234 remaining after 0.5 years:
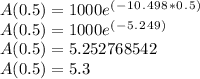
Thus, the amount of Thorium-234 remaining after 0.5 years is approximately 5.3 grams.
(b) We plug in 115 for A(t) and 1000 for A0. Then, we must solve for t: Thus, our equation to solve for t, time in years, is
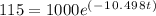
Step 1: Divide both sides by 1000.
115/1000 = e^(-10.498t)
0.115 = e^(-10.498t)
Step 2: Take the natural log (ln) of both sides.
ln(0.115) = ln(e^(-10.498t))
Step 3: According to the rules of natural logs, we can bring -10.498t down and multiply it by ln(e) on the right-hand side of the equation:
ln(0.115) = -10.498t * ln(e)
ln(0.115) = -10.498t * 1
ln(0.115) = -10.498t
Step 4: Now, we divide both sides by -10.498 and round the result to find out after about how many years will 115 grams of thorium-234 be remaining:
(ln(0.115) = -10.498t) / -10.498
0.2060223996 = t
0.2 = t
Thus, there will be 115 grams of thorium-234 remaining after about 0.2 years