Answer:
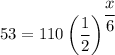
Explanation:
The decay of a radioactive substance can be modeled using the half-life formula:
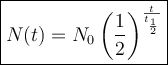
where:
- N(t) is the quantity of the substance remaining after time t.
- N₀ is the initial quantity of the substance.
- t is the time elapsed.
- t_{1/2} is the half-life of the substance.
In this case, the initial amount of substance is N₀ = 110 grams and the half-life is t_{1/2} = 6 days.
To find the number of days, x, required for the substance to decay to 53 grams, we can set N(t) = 53 and t = x.
Therefore, the equation that can be used to determine the number of days, x, required for the substance to decay to 53 grams is:
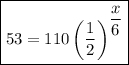

To solve the equation, divide both sides by 110:
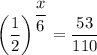
Take natural logarithms of both sides:
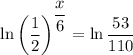
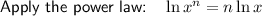
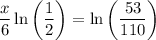
Multiply both sides by 6:
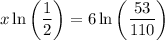
Divide both sides by ln(1/2):
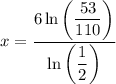
Evaluate:
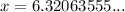
Therefore, the number of days required for the substance to decay to 53 grams is approximately 6.32 days, as calculated using the half-life formula.