Answer:
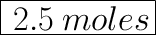
Step-by-step explanation:
The number of moles of
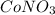 solution given its volume and concentration can be found by using the formula,
solution given its volume and concentration can be found by using the formula,

where
c is the concentration in M , mol/dm³ or mol/L
v is the volume in L or dm³
n is the number of moles
From the question.
v = 5 L
c = 0.5 M
Doing a change of subjects we have,
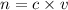
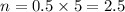
We have the final answer as
2.5 moles