Answer:
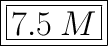
Step-by-step explanation:
The molarity of sodium sulfate given its volume and number of moles can be found by using the formula;

where
c is the concentration in M , mol/dm³ or mol/L
v is the volume in L or dm³
n is the number of moles
From the question.
n = 0.09 moles
v = 12 ml
Using the conversion,
1000 ml = 1 L
12 ml = 0.012 L
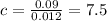
We have the final answer as
7.5 M