Answer:
( I only know 2,3,4 )
If we start this reaction with 40 grams of magnesium and an excess of nitric acid, how many grams of hydrogen gas could be produced?
ANSWER: 40.g Mg + 1 mol HNO3 à 1 mol Mg (NO3)2+ 2.02g H2
= 3.3g H2
If 1.7 grams of hydrogen is actually produced, what was the percent yield of hydrogen?
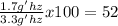
ANSWER:
Magnesium metal reacts quantitatively with oxygen to give magnesium oxide, MgO. If 5.00 g of Mg and 5.00 g of O2 are allowed to react, what weight of MgO is formed, and what weight of which reactant is left in excess?
ANSWER: 2 Mg(s) + O2(g) → 2 MgO(s)