Answer:
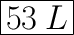
Step-by-step explanation:
Since temperature is constant the new volume can be found by using the formula for Boyle's law which is;
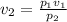
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
From the question
v1 = 37 L
P1 = 1 atm
P2 = 0.7 atm
Substituting the values into the above formula we have.
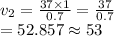
We have the final answer as
53 L