Answer: The volume of oxygen that would be needed to completely burn
75.0 liters of acetylene
 at STP is 187.5 liters.
at STP is 187.5 liters.
Definition of volume : -Volume is the amount of space occupied by a substance, while mass is the amount of matter it contains. The units of volume are liter, milliliter,
Definition of STP : Standard temperature and pressure (STP) refers to the nominal conditions in the atmosphere at sea level. These conditions are 0 degrees Celsius and 1 atmosphere (atm) of pressure. The STP value is important to physicists, chemists, engineers, pilots and navigators, among others.
Explanation: Given : Volume of acetylene
 =
=
 liters.
liters.
When oxygen is burnt in acetylene , the chemical reaction is given as:
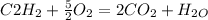
So, for
 liters of acetylene Volume of oxygen required =
liters of acetylene Volume of oxygen required =
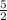 ×
×
 =
=
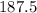 liters.
liters.
Final answer : The volume in liters of oxygen that would be needed to completely burn 75.0 liters of acetylene
 at STP is 187.5 liters
at STP is 187.5 liters