Answer:
It increases by a factor of four.
Step-by-step explanation:
We can use the combined gas law
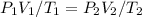 .
.
Based on the question, we're looking for
 . The formula rewritten for
. The formula rewritten for
 :
:
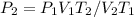 .
.
We know the volume stays constant, so
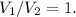 Our new formula:
Our new formula:
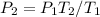 .
.
We know that
 so plugging that into the equation gives us:
so plugging that into the equation gives us:
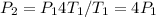 .
.
Therefore, the pressure of the gas increases by a factor of four.