Answer:
0.072 J/kg*K
Known Equation
Specific Heat [
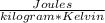 ] = c =
] = c =
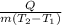
where Q [J] = Heat, m [kg] = mass, and ΔT [K] = change in temperature
Given Variables
Q = 200 J
m = 10 g
ΔT = 5 °C = 5 °C + 273 = 278 K
Solve
Specific Heat = c = (200 J) / (10 g * 278 K) = 0.072 J/kg*K