Answer:
2.07 M
Step-by-step explanation:
Given,
Previous Volume,
 = 345 ml ; Previous molarity,
= 345 ml ; Previous molarity,
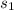 = 1.5 M
= 1.5 M
Current Volume,
 = 250 ml
= 250 ml
Molar mass of NaCl, M = (23+35.5)=58.5 g
Now,
mass of NaCl present in the solution, w =

= 30.27375 g
Now,
Changed Molarity,
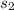 =
=
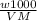
= 2.07 M