Final answer:
The number of shared electrons (S) in silicon tetrachloride
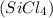 is calculated to be 24, given the total number of valence electrons (N = 32) and the number of electrons needed for a full octet around each atom (A = 8).
is calculated to be 24, given the total number of valence electrons (N = 32) and the number of electrons needed for a full octet around each atom (A = 8).
Step-by-step explanation:
To find the number of shared electrons, denoted as S, in silicon tetrachloride
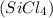 , you first need to determine the total number of valence electrons that should be present in the compound (N) and the number of electrons needed to give each atom in the compound an octet (A). Silicon (Si) having 4 valence electrons and each chlorine (Cl) having 7 valence electrons gives us a total of N = 4 + (7×4) = 32 valence electrons for
, you first need to determine the total number of valence electrons that should be present in the compound (N) and the number of electrons needed to give each atom in the compound an octet (A). Silicon (Si) having 4 valence electrons and each chlorine (Cl) having 7 valence electrons gives us a total of N = 4 + (7×4) = 32 valence electrons for
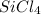 . Since silicon tetrachloride is a covalent tetrahedral molecule, silicon is at the center with four chlorine atoms surrounding it, forming single bonds. Each Si-Cl bond requires 2 electrons so for four bonds, A = 2×4 = 8.
. Since silicon tetrachloride is a covalent tetrahedral molecule, silicon is at the center with four chlorine atoms surrounding it, forming single bonds. Each Si-Cl bond requires 2 electrons so for four bonds, A = 2×4 = 8.
Therefore, S = N - A = 32 - 8 = 24 shared electrons in
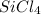 . Silicon tetrachloride is also known as a nonpolar, low-boiling, colorless liquid with a boiling point of 57 °C.
. Silicon tetrachloride is also known as a nonpolar, low-boiling, colorless liquid with a boiling point of 57 °C.