(a)
Before the addition of any acid, we just treat this as a weak base problem, dealing with just the ionization of the weak base ammonia.
The ionization of ammonia is expressed by this reaction:
NH₃(aq) + H₂O(l) ⇄ OH⁻(aq) + NH₄⁺(aq)
We can set up an ICE table to show the initial concentration of each reactant and product, the change in concentration, and the concentration of all at equilibrium.
⇒NH₃(aq) + H₂O(l) ⇄ OH⁻(aq) + NH₄⁺(aq)
I 0.1 0 0
C -x +x +x
E 0.1 -x x x
(note that water is a liquid and therefore has no concentration)
We know that
![k_b = ([products])/([reactants])](https://img.qammunity.org/2024/formulas/chemistry/high-school/3flmbi3esko81be27apf0v19fusy6zgo3g.png) and we know the value of kb given, so
and we know the value of kb given, so
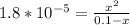
We could just solve for x from here, however that would end up being a quadratic equation which are annoying.
Since ammonia is a weak base, we can assume the amount of ammonia used (x) will be a negligible amount, and drop the -x from 0.1-x.
The statement now becomes
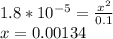
So, the concentration of OH⁻ = 0.00134 M
We can find the pOH from this, as pOH = -log([OH⁻])
pOH = -log(0.00134) = 2.872
pH = 14-pOH = 11.1
So, pH = 11.13
(b)
Before the equivalence point (when moles of base equal the moles of added acid), we are dealing with a buffer solution and can treat it as such.
The equation we will use is
NH₃(aq) + H₃O⁺(aq) → NH₄⁺(aq) + H₂O
After 20 ml of 0.1 M HCl has been added, 0.002 moles of HCl have been added.
There are two ways to do this, and I will do both.
Here I set up a mole table showing the moles of each reactant and product before and after reaction.
The H⁺ ions are given by the acid, so the moles of H⁺ will equal moles of acid.
⇒ NH₃(aq) + H⁺(aq) → NH₄⁺(aq)
before 0.005 0.002 0
after 0.003 0 0.002
H⁺ is the limiting reactant, so H⁺ will be completely used up and the remaining moles of NH₃ will be subtracted by that amount and NH₄⁺ will be produced by that amount.
From here, you must choose which method to do. Personally I find method 2 easier.
METHOD 1
Returning to this equation:
NH₃(aq) + H₂O(l) ⇄ OH⁻(aq) + NH₄⁺(aq)
we can plug in the new initial value of NH₄⁺ gotten from the reaction between NH₃(aq) and H⁺(aq). Set up another ICE table with this new initial concentration. NOTE that we have 0.002 moles of NH₄⁺ and 0.005 moles of NH₃ initially, but that is not concentration. We have to put these values over the new volume (0.02 + 0.05 ) to find concentrations.
⇒NH₃(aq) + H₂O(l) ⇄ OH⁻(aq) + NH₄⁺(aq)
I 0.0429 0 0.0286
C -x +x +x
E 0.0429 -x x 0.0286+x
![k_b = ([products])/([reactants])](https://img.qammunity.org/2024/formulas/chemistry/high-school/3flmbi3esko81be27apf0v19fusy6zgo3g.png)
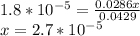 again assuming the change in concentration of NH₃ is negligible.
again assuming the change in concentration of NH₃ is negligible.
pOH = -log(x) = 4.568
pH = 14 - pOH = 9.43
METHOD 2
With the moles of NH₃ and its conjugate acid, NH₄⁺, we can plug them into the Henderson-Hasselbalch equation since it is a buffer solution before it hits the equivalence point.
The Henderson-Hasselbalch equation is
![pH = pKa + log([A^-])/([HA])](https://img.qammunity.org/2024/formulas/chemistry/high-school/ximm9bfod5bwk8iocrb7xl6t6599n37i6x.png)
for an acid and
![pOH = pKb + log([BH^+])/([B])](https://img.qammunity.org/2024/formulas/chemistry/high-school/lwbbhr62z0zqhnpu0tu1196iiz0o09iey3.png)
for a base, where B is the base and BH+ is its conjugate acid. While the equation uses the concentrations of each, we can just use moles.
note that pKb = -log(kb)
Since this is a base, we will use the second equation.
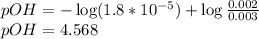
pH = 14 - pOH = 9.43
(c)
After half of the NH₃ is neutralized, that means we are halfway to the equivalence point. At halfway to the eq. point, pOH = pkb and pH = pka
So, pOH = -log(kb) = 4.74
pH = 14 - pOH = 9.26
(d)
At the equivalence point, moles of base and added acid are the same.
⇒ NH₃(aq) + H⁺(aq) → NH₄⁺(aq)
before 0.005 0.005 0
after 0 0 0.005
Only NH₄⁺ remains, so this is just a weak acid ionization problem.
Take the moles of NH₄⁺ and put it over the total volume--
Since we have 0.005 moles of 0.1 M HCl, we have 50 mL of HCl and 50 mL of NH₃, so 100 mL or 0.1 L total.
Another ICE table!
⇒ NH₄⁺(aq) + H₂O(l) ⇄ H₃O⁺(aq) + NH₃(aq)
I 0.05 M 0 0
C -x +x +x
E 0.05-x x x
Now to find ka.
ka*kb = kw
kw is a constant,
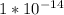
So,
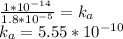
Back to the ice table.
![k_a = ([products])/([reactants])\\k_a = (x^2)/(0.05)\\](https://img.qammunity.org/2024/formulas/chemistry/high-school/29khdcyhtfurvc3xz7nk7e45ueobt2pi3x.png) again, assuming the ionization of NH₄⁺ is negligible
again, assuming the ionization of NH₄⁺ is negligible
Solving for x, we get x=5.270
x in this case is the concentration of H₃O⁺, so -log(x) = pH
pH = -log(5.270) = 5.28
Hope I could help!