Answer:
Approximately
 .
.
Step-by-step explanation:
By the Heisenberg Uncertainty Principle:
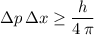 ,
,
Where:
 is the uncertainty in momentum,
is the uncertainty in momentum,
 is the uncertainty in position, and
is the uncertainty in position, and
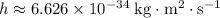 is Planck's Constant.
is Planck's Constant.
In this question, the measured velocity of this electron is
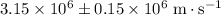 . The uncertainty of this measurement is
. The uncertainty of this measurement is
 .
.
Assume that the mass of this electron is the same as that of a stationary electron:
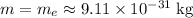 .
.
With an uncertainty in velocity of
 , the uncertainty in the momentum of this electron would be:
, the uncertainty in the momentum of this electron would be:
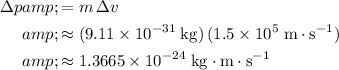 .
.
Rearrange the Uncertainty Principle to find the uncertainty in position:
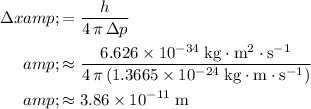 .
.