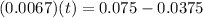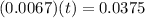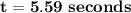Answer:
Step-by-step explanation:
From the given information:
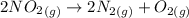
The above reaction is a zero-order reaction.
The rate constant = 0.0067 M-s⁻¹
Suppose the volume of the flask = 4L
Initial Mol of dinitrogen monoxide = 300 mmol
The final mol of dinitrogen monoxide = 150 mmol
The molarity of dinitrogen monoxide =
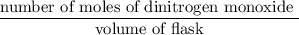
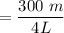
= 0.0075 mmol/L
= 0.0075 L
The final concentration
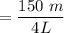
= 0.0375 L
By applying zero order equation
![kt = [A_o] -[At]](https://img.qammunity.org/2022/formulas/chemistry/college/ti629vdvkrxjg3rrp69t27arz5a4mte029.png)