Answer: 2.53
Step-by-step explanation: The ideal gas equation is formulated as:

P = Pressure of the ideal gas,
V = Volume of the ideal gas,
n = Total amount of ideal gas that is measured in terms of moles,
R = Gas constant, and
T = Temperature
By rearranging the ideal gas equation we get,
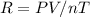
P = 380 kPa
V = 6 L
n = 3 moles
T = 27+273 = 300 K
Put the value given in this equation:
R = 380 kPa . 6L / 3 mol . 300 K
R = 2.53