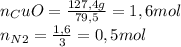Answer:
1) 234,0 g 2) 7,5 3) 0,5
Step-by-step explanation:
1) Since the stoichiometric coefficients of carbon and methane are equal, the moles of carbon needed are the same as the moles of methane produced. Therefore the mass of carbon needed can be calculated as follows
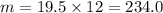
2)This exercise is comparable to the former one. Since the stoichiometric coefficients are the same, the moles of methane procured and the moles of the carbon needed are the same.
The molar mass of methane is 12 + 1 × 4 = 16

3) In this case, the stoichiometric coefficients are not equal. In order to produce 1 mole of nitrogen, 3 moles of CuO (copper (II) oxide) are needed. Therefore, the number of moles of CuO consumed must be divided by 3 in order to get the moles of nitrogen produced.
Molar mass of CuO = 63,5 + 16 = 79.5