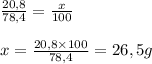Step-by-step explanation:
The Molar mass of K2O is 94,2 g/mol
The moles of K2O can be calculated
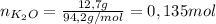
The stoichiometric coefficients must be considered: in order to produce 3 moles of K2O, 1 mole of Pb2O3 are needed. In other words, if 1 mole of K2O is produced, 1/3 of a mole of Pb2O3 is needed. So
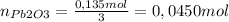
The molar mass of Pb2O3 is 462,4 g/mol
The theoretical mass of Pb2O3 used to produce K2O is
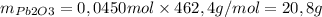
Since the yield is not 100%, a larger mass was needed to perform the reaction.