Answer:
Explanation: Molarity= Number of moles of solute
Volume of solution in liters
given,
mass of solute, NaCl= 40g
volume of solution = 500ml = 0.5L
number of moles of solute = mass in grams
molecular mass
Molecular mass of NaCl = 23*1 + 35.5*1
= 23 + 35.5
= 58.5g
no. of moles = 40/58.5
= 0.68 mol
molarity = 0.68/0.5
=
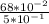
=
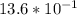
= 1.36 M