Answer:
The pressure changes from 2.50 atm to 2.58 atm (an increase of approximately 0.08 atm) when the gas is heated from 30.0°C to 40.0°C.
Step-by-step explanation:
As the given volume of gas is constant, we can use Gay-Lussac's law to solve this problem as it relates pressure to temperature.
Gay-Lussac's law

where:
- P₁ = Initial pressure
- T₁ = Initial temperature (in kelvins)
- P₂ = Final pressure
- T₂ = Final temperature (in kelvins)
First, we need to convert the given temperatures from Celsius to Kelvin by adding 273.15:
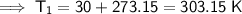
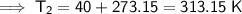
Therefore, the values to substitute into the equation are:
- P₁ = 2.50 atm
- T₁ = 303.15 K
- T₂ = 313.15 K
As we are solving for the final pressure, rearrange the equation to isolate P₂:
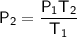
Substitute the values into the equation and solve for P₂:
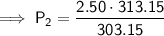
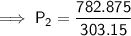
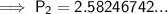
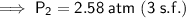
Therefore, the pressure changes from 2.50 atm to 2.58 atm (an increase of approximately 0.08 atm) when the gas is heated from 30.0°C to 40.0°C.