Answer:
3.59 mol (3 s.f.)
Step-by-step explanation:
To determine the number of moles of gas in the canister, we can use the Ideal Gas Law.
Ideal Gas Law
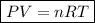
where:
- P is the pressure measured in atmosphere (atm).
- V is the volume measured in liters (L).
- n is the number of moles.
- R is the ideal gas constant (0.082057366080960 atm L mol⁻¹ K⁻¹).
- T is the temperature measured in kelvin (K).
As the given pressure is in torr, we need to convert it to atmospheres (atm). As 1 atm = 760 torr, to convert torr to atm, divide the pressure value by 760:
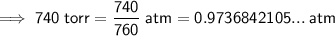
Therefore, the values to substitute into the equation are:
- P = 0.9736842105 atm
- V = 62 L
- R = 0.082057366080960 atm L mol⁻¹ K⁻¹
- T = 205 K
As we want to find the number of moles, rearrange the equation to isolate n:
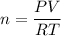
Substitute the values into the equation and solve for n:
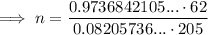
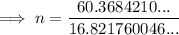
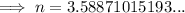
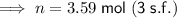
Therefore, the number of moles of gas in the canister is 3.59 moles (rounded to three significant figures).