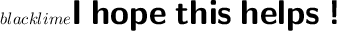$\leadsto\sf\textbf\:pH\:\:=\:-\log[H^+]$
$\leadsto\sf\textbf\:[H^+]\:\:=\:10^{-pH}$
For the buffer to have a pH of 3.5, we want the concentration of H+ ions to be $\sf\textbf\;3.16\:x\:10^-4:\:M.$
Using the Henderson-Hasselbalch equation:
$\leadsto\sf\textbf\:pH\:\:=\:pKa\:+\:\log\frac{[A^-]}{[HA]}$
$\leadsto\sf\textbf\:3.5\:\:=\:pKa\:+\:\log\frac{[A^-]}{[HA]}$
$\leadsto\sf\textbf\:log\frac{[A^-]}{[HA]}\:\:=\:3.5\:-\:pKa$
$\leadsto\sf\textbf\:\frac{[A^-]}{[HA]}\:\:=\: 10^{3.5-pKa}$
Comparing the pKa values of formic acid and acetic acid, we see that formic acid has a lower pKa value, which means it is a stronger acid than acetic acid. Therefore, formic acid is a better choice for the buffer, as it will donate more H+ ions to the solution, leading to a more stable pH.
NaOH is added to the buffer to act as a base, which will react with any excess acid that is added to the solution. This prevents the pH from dropping too low and becoming too acidic. The NaOH reacts with the H+ ions to form water, effectively removing excess H+ ions from the solution.