The pH of a 287 M aqueous solution of ammonium iodide is approximately 5.51, indicating that the solution is acidic.
The pH of a solution can be calculated using the concentration of hydrogen ions
 in the solution. Ammonium iodide
in the solution. Ammonium iodide
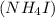 is a salt that dissociates in water into its constituent ions: ammonium
is a salt that dissociates in water into its constituent ions: ammonium
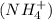 and iodide
and iodide
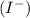 ions.
ions.
The ammonium ion
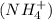 is acidic because it can donate a proton
is acidic because it can donate a proton
 in water:
in water:
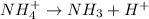
The concentration of
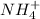 ions in a 287 M solution of ammonium iodide is 287 M. To find the pH, you can calculate the concentration of
ions in a 287 M solution of ammonium iodide is 287 M. To find the pH, you can calculate the concentration of
 ions produced by the dissociation of
ions produced by the dissociation of
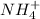 .
.
However, to accurately calculate the pH, you need to consider the dissociation constant
 of ammonium ion
of ammonium ion
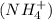 in water, which is approximately
in water, which is approximately
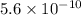 . Using this dissociation constant, you can calculate the concentration of
. Using this dissociation constant, you can calculate the concentration of
 ions formed from the dissociation of
ions formed from the dissociation of
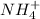 .
.
Let's calculate the pH:
Given: Concentration of
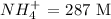
The equation for the dissociation of ammonium ion is:
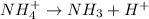
The
 expression for this dissociation is:
expression for this dissociation is:
![\(K_a = ([NH_3][H^+])/([NH_4^+]) = 5.6 * 10^(-10)\)](https://img.qammunity.org/2024/formulas/chemistry/high-school/sf8sh636fleeplabqi3jgsc074psu1cvwq.png)
Given that the initial concentration of
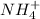 is 287 M and assuming negligible contribution from water:
is 287 M and assuming negligible contribution from water:
Let x be the concentration of
 ions formed. Since the dissociation is 1:1:
ions formed. Since the dissociation is 1:1:
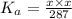
Solving for x:
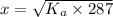
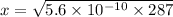
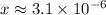
So, the concentration of
 ions formed from the dissociation of
ions formed from the dissociation of
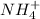 is approximately
is approximately
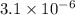 M.
M.
Using the definition of pH:
![\(pH = -\log[H^+]\)](https://img.qammunity.org/2024/formulas/chemistry/high-school/c15ddr4ybwnsdzstubrilc398dogulobvh.png)
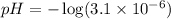
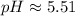
Therefore, The answer is approximately 5.51.
The complete question is here:
What is the PH of a 287 M м aqueous solution of ammonium Iodide, NH 4 I ? PH : is the solution (acidic, basic, neutral)