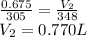Answer: 770 mL
Step-by-step explanation:
Charles' law states that
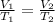 , so as temperature increases, volume does as well. We can plug in our values for V₁,T₁,and T₂ to this equation and solve for V₂, using L for volume and, importantly, kelvin for temperature. (kelvin is 273 + celsius).
, so as temperature increases, volume does as well. We can plug in our values for V₁,T₁,and T₂ to this equation and solve for V₂, using L for volume and, importantly, kelvin for temperature. (kelvin is 273 + celsius).