This question is incomplete, the complete question is;
A monatomic gas fills the left end of the cylinder in the following figure. At 300 K , the gas cylinder length is 14.0 cm and the spring is compressed by65.0 cm . How much heat energy must be added to the gas to expand the cylinder length to 16.0 cm ?
Answer:
the required heat energy is 16 J
Step-by-step explanation:
Given the data in the question;
Lets consider the ideal gas equation;
PV = nRT
from the image, we calculate initial pressure;
Pi = ( 2000N/M × 0.06m) / 0.0008 m²
Pi = 15 × 10⁴ Pa
next we find Initial velocity
Vi = (0.0008 m²)(0.14) = 1.1 × 10⁻⁴ m²
now we find the number of moles
n = [(15 × 10⁴ Pa)(1.1 × 10⁻⁴ m²)] / 8.31 J/molK × 300K
N = 6.6 × 10⁻³ mol
next we calculate the final temperature;
Pf = ( 2000N/m × 0.08) / 0.0008 m²
Pf = 2 × 10⁵ Pa
Calculate the final Volume
Vf = (0.0008 m² × 0.16 m = 1.28 × 10⁻⁴ m³
we also determine the final temperature
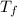 = (2 × 10⁵ Pa × 1.28 × 10⁻⁴ m³) / 6.6 × 10⁻³ × 8.31 J/molK
= (2 × 10⁵ Pa × 1.28 × 10⁻⁴ m³) / 6.6 × 10⁻³ × 8.31 J/molK
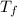 = 466.8 K
= 466.8 K
so change in temperature ΔT
ΔT = 466.8 K - 300K = 166.8 K
we then calculate the change in thermal energy
ΔU = nCΔT
ΔU = ( 6.6 × 10⁻³ mol ) × 12.5 × 166.8K
ΔU = 13.761 J
C is the isochoric molar specific heat which is equal to 3R/2 for monoatomic
now we calculate the work done;
W = 1/2 × K( x
 ² - x
² - x
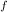 ² )
² )
W = 1/2 × ( 2000 N/m) ( 0.06² - 0.08² )
= - 2.8 J
and we then calculate the heat energy using the following expression;
Q = ΔU - W
we substitute
Q = 13.761 - (- 2.8 J)
Q = 13.761 + 2.8 J)
Q = 16 J
Therefore, the required heat energy is 16 J