Answer:
Step-by-step explanation:
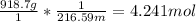 To start off the mol of HgO must be found.
To start off the mol of HgO must be found.
After that the molar ratio between HgO and O must be found but in this case its 1:1
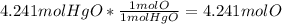 the mols of HgO is put on the bottom to cancel out with the other one leaving just mols of oxygen. Finally to find g of oxygen it must be multiplied by its molar mass.
the mols of HgO is put on the bottom to cancel out with the other one leaving just mols of oxygen. Finally to find g of oxygen it must be multiplied by its molar mass.
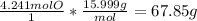 Oxygen
Oxygen