Answer:
630.95 grams of Na₂CO₃ would be needed to produce 1000g of NaHCO₃
Step-by-step explanation:
The balanced reaction is:
Na₂CO₃ + CO₂+ H₂O → 2 NaHCO₃
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
- Na₂CO₃: 1 moles
- CO₂: 1 mole
- H₂O: 1 mole
- NaHCO₃: 2 moles
Being the molar mass:
- Na₂CO₃: 106 g/mole
- CO₂: 44 g/mole
- H₂O: 18 g/mole
- NaHCO₃: 84 g/mole
Then by stoichiometry the following quantities of mass participate in the reaction:
- Na₂CO₃: 1 mole* 106 g/mole= 106 g
- CO₂: 1 mole* 44 g/mole= 44 g
- H₂O: 1 mole* 18 g/mole= 18 g
- NaHCO₃: 2 moles* 84 g/mole= 168 g
You can apply the following rule of three: if 106 grams of Na₂CO₃ are needed to produce 168 grams of NaHCO₃, how much mass of Na₂CO₃ is necessary to produce 1000 grams of NaHCO₃?
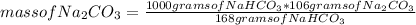
mass of Na₂CO₃= 630.95 grams
630.95 grams of Na₂CO₃ would be needed to produce 1000g of NaHCO₃