Answer:
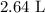
Step-by-step explanation:
Assuming that the helium is at standard temperature and pressure (STP), we can use the molar volume of an ideal gas at STP (22.4L) to solve for its volume.
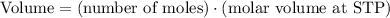
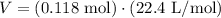
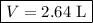
Note that we rounded to 3 significant figures because that is how many significant figures were given by the measurement of helium (0.118 mol).