Answer:
1.39 mL
Step-by-step explanation:
To determine the volume of 18.0 Molarity
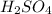 needed to contain 2.45 grams of
needed to contain 2.45 grams of
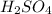 , we can use the following formula:
, we can use the following formula:
moles of solute = mass of solute / molar mass of solute
Then, we can use the molarity formula:
Molarity = moles of solute / volume of solution (in liters)
Rearranging this formula, we get:
volume of solution (in liters) = moles of solute / Molarity
First, we need to calculate the moles of
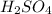 :
:
moles of
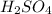 = mass of
= mass of
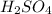 / molar mass of
/ molar mass of
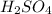
The molar mass of
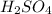 is:
is:
2(1.008 g/mol) + 32.06 g/mol + 4(16.00 g/mol) = 98.08 g/mol
Therefore, the moles of
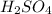 are:
are:
moles of
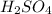 = 2.45 g / 98.08 g/mol = 0.02497 mol
= 2.45 g / 98.08 g/mol = 0.02497 mol
Next, we can calculate the volume of 18.0 Molarity
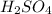 needed:
needed:
volume of solution (in liters) = moles of solute / Molarity
volume of solution (in liters) = 0.02497 mol / 18.0 mol/L = 0.001387 L
Finally, we can convert the volume to milliliters:
volume of solution (in mL) = 0.001387 L x 1000 mL/L = 1.39 mL
Therefore, approximately 1.39 mL of 18.0 Molarity
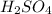 is needed to contain 2.45 grams of
is needed to contain 2.45 grams of
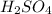 .
.