Answer:
0.01 M
Step-by-step explanation:
NaOH (sodium hydroxide) is a strong base, so it is completely dissociated into ions in aqueous solution:
NaOH ⇄ Na⁺ + OH⁻
So, we have OH⁻ ions in solution.
From the problem, we have the pH value. The pH is calculated as:
pH = -log [H⁺]
As NaOH is a base, we can also calculate the pOH:
pOH = -log [OH⁻]
Thus. we can use the relation between pH and pOH to calculate the pOH and then use it to calculate [OH⁻]:
pH + pOH = 14 ⇒ pOH = 14 - pH = 14 - 12.05 = 1.95
pOH = -log [OH⁻] ⇒ [OH⁻] =
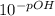 =
=
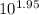 = 0.0112 M ≅ 0.01 M
= 0.0112 M ≅ 0.01 M