Charles's Law-
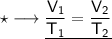
Where:-
- V₁ = Initial volume
- T₁ = Initial temperature
- V₂ = Final volume
- T₂ = Final temperature
As per question, we are given that -
- V₁=425 mL
- T₁ = 250°C
- T₂ =125°C
We are given the initial temperature and the final temperature in °C.So, we first have to convert those temperatures in Celsius to kelvin by adding 273-
 = 250+ 273 = 523 K
= 250+ 273 = 523 K
 =125+273 = 398K
=125+273 = 398K
Now that we have obtained all the required values, so we can put them into the formula and solve for V₂ :-
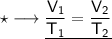
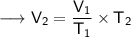
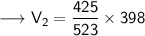
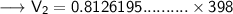
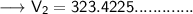
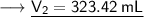
Therefore, the volume of this gas at 125°C will become 323.42 mL.