To find the volume of ethane , we can use the Ideal gas law. Which states -

Where:-
- P is the pressure measured in atmospheres
- V is the volume measured in liters
- n is the number of moles.
- R is the ideal gas constant (0.0821 L atm mol⁻¹ K⁻¹).
- T is the temperature measured in kelvin.
As per question, we are given that-
- P=0.9035 atm
- n=5.2643 moles
- T = 506.25°C = 506.25+273 = 779.25 K
- R = 0.0821 L atm mol⁻¹ K⁻¹
Now that we have all the required values, so we can put them all in the Ideal gas law formula and solve for Volume -

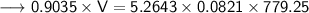
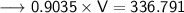
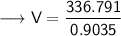
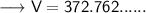
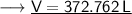
- Therefore, the volume of ethane is 372.762 L.