Answer:
No, the given equation is not balanced. The balanced equation is:
SrBr₂ + (NH₄)₂CO₃ → SrCO₃ + 2NH₄Br
Step-by-step explanation:
A chemical equation is a representation of a chemical reaction using chemical formulas and symbols. It shows the reactant(s) on the left side of the equation and the product(s) on the right side of the equation, separated by an arrow that indicates the direction of the reaction.
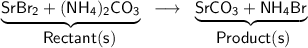
A balanced chemical equation has the same number of atoms of each element on both sides of the equation.
Coefficients are used to balance chemical equations and are placed in front of a chemical symbol or formula where needed.
Given chemical equation:

Here, we need to balance the number of Sr, Br, N, H, C, and O atoms.
Check to see if there are the same number of atoms of each element on both sides of the equation:
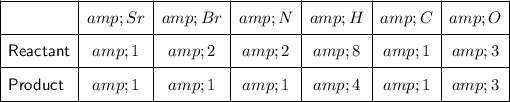
We can see that there are two bromine atoms on the left but only one on the right, so a coefficient of 2 needs to be added to NH₄Br on the right side of the equation:

By adding the coefficient 2 to NH₄Br on the right side of the equation, the number of N, H and Br atoms on this side have been multiplied by 2. So we now have:
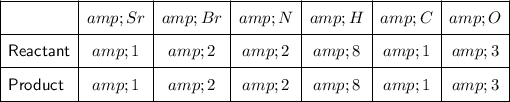
As there are now the same number of atoms of each element on both sides of the equation, it is balanced.
Therefore, the balanced chemical equation is:
