Answer:
Approximately
 .
.
Step-by-step explanation:
Initial volume of the solution:
 .
.
Initial quantity of
 :
:
 .
.
Ammonia
 reacts with hydrochloric
reacts with hydrochloric
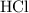 acid at a one-to-one ratio:
acid at a one-to-one ratio:
 .
.
Hence, approximately
 of
of
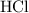 molecules would be required to exactly react with the
molecules would be required to exactly react with the
 in the original solution and hence reach the equivalence point of this titration.
in the original solution and hence reach the equivalence point of this titration.
Calculate the volume of that

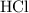 solution required for reaching the equivalence point of this titration:
solution required for reaching the equivalence point of this titration:
 .
.
Hence, by the assumption stated in the question, the volume of the solution at the equivalence point would be approximately
 .
.
If no hydrolysis took place,
 of
of
 would be produced. Because
would be produced. Because
 is a soluble salt, the solution would contain
is a soluble salt, the solution would contain
 of
of
 ions. The concentration of
ions. The concentration of
 would be approximately:
would be approximately:
 .
.
However, because
 is a weak base, its conjugate
is a weak base, its conjugate
 would be a weak base.
would be a weak base.
 .
.
Hence, the following reversible reaction would be take place in the solution at the equivalence point:
 .
.
Let
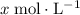 be the increase in the concentration of
be the increase in the concentration of
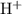 in this solution because of this reversible reaction. (Notice that
in this solution because of this reversible reaction. (Notice that
 .) Construct the following
.) Construct the following
 table:
table:
 .
.
Thus, at equilibrium:
- Concentration of the weak acid:
![[{\rm {NH_4}^(+)}] \approx (0.255782 - x) \; \rm M](https://img.qammunity.org/2022/formulas/chemistry/college/berolumz8n4s6v0nwlk7mhygwy8s4aa1ef.png) .
. - Concentration of the conjugate of the weak acid:
![[{\rm NH_3}] = x\; \rm M](https://img.qammunity.org/2022/formulas/chemistry/college/7b9g2qowvct5w10su4pvjpf109pvd3gxz8.png) .
. - Concentration of
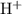 :
:
![[{\rm {H}^(+)}] \approx x\; \rm M](https://img.qammunity.org/2022/formulas/chemistry/college/9m7pyt27skswp3353jftcmfzrxqy1zo8s1.png) .
.
![\displaystyle \frac{[{\rm NH_3}] \cdot [{\rm H^(+)}]}{[{ \rm {NH_4}^(+)}]} = 10^{pK_\text{a}({\rm {NH_4}^(+)})}](https://img.qammunity.org/2022/formulas/chemistry/college/1eolkf4xdsy0pn0wo63q576ysfqu6epnwy.png) .
.

Solve for
 . (Notice that the value of
. (Notice that the value of
 is likely to be much smaller than
is likely to be much smaller than
 . Hence, the denominator on the left-hand side
. Hence, the denominator on the left-hand side
 .)
.)
 .
.
Hence, the concentration of
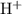 at the equivalence point of this titration would be approximately
at the equivalence point of this titration would be approximately
 .
.
Hence, the
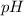 at the equivalence point of this titration would be:
at the equivalence point of this titration would be:
![\begin{aligned}pH &= -\log_(10)[{\rm {H}^(+)}] \\ &\approx -\log_(10) \left(1.19929 * 10^(-5)\right) \approx 4.92\end{aligned}](https://img.qammunity.org/2022/formulas/chemistry/college/4lp2mvbpieokf5zza1rcgmi91jytfzxahc.png) .
.