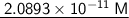We know that pH is a measure of acidity or alkalinity that indicates the amount of hydrogen ions present in a solution or substance which is defined as the negative base 10 logarithm of the activity of hydrogen ions. Which states -
- pH= - log [H⁺]= - log [H₃O⁺]
As per question, we are given a pH value which is 10.68 for a solution and have asked to find out the hydronium ion concentration. Since we have the value of pH, so we can put into the formula and solve for [H₃O⁺] -
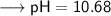
![\:\:\:\:\:\:\longrightarrow \sf - log [H₃O⁺] = 10.68 \\](https://img.qammunity.org/2024/formulas/chemistry/college/ixr7suv85ymxycjs79wvl4xx02dr7nu3ui.png)
![\:\:\:\:\:\:\longrightarrow \sf [H₃O⁺] = 10^(-10.68)\\](https://img.qammunity.org/2024/formulas/chemistry/college/3in645e627bn032hamtlowypels7rh1g9d.png)
![\:\:\:\:\:\:\longrightarrow \sf \underline{[H₃O⁺] = 2.0893 * 10^(-11)\:M} \\](https://img.qammunity.org/2024/formulas/chemistry/college/cxoqlnw5gebawaao2ki2ifizwvoy184xho.png)
Therefore, the hydronium concentration for the solution is