Answer:
16.1 g
Step-by-step explanation:
We want to find how many grams of NO₂ can be produced from 15.0 g of NO and 5.60 g of O₂ according to the balanced chemical equation:
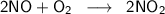
First, convert the given masses of both reactants, NO and O₂, to moles using their respective relative formula masses
 .
.
Relative formula masses:
Therefore:
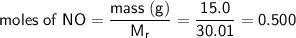
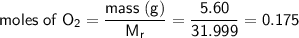
Now look at the ratio of the reactants:
2 mol NO : 1 mol O₂ = 0.5 mol NO : 0.25 mol O₂
There are only 0.175 moles of O₂ (instead of 0.25 moles), so the O₂ will run out first. It is the limiting reactant.
Use the moles of the limiting reactant to calculate the mass of the product, remembering to use the molar ratio between the limiting reactant and the product.
Limiting reactant : product = 1 mol O₂ : 2 mol NO₂
Therefore, 0.175 mol O₂ will make 0.35 mol NO₂.
Finally, convert the moles of NO₂ to grams:
