Boyle's Law-

(Pressure is inversely proportional to the volume)
Where-
 = Initial volume
= Initial volume
 = Final volume
= Final volume
 = Initial pressure
= Initial pressure
 = Final pressure
= Final pressure
As per question, we are given that -
 = 160 cm³
= 160 cm³
 = 100KPa
= 100KPa
 = 80KPa
= 80KPa
Now that we have all the required values and we are asked to find out volume which will be occupied if the pressure is adjusted to 80 KPa and the temperature remains unchanged. For that we can put the values and solve for the final volume of helium-
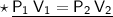
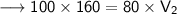

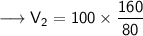

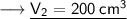
- Therefore, 200 cm³ will be occupied if the pressure is adjusted to 80 KPa and the temperature remains unchanged.