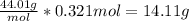Answer: 14.11 g
Step-by-step explanation:
Ideal gas law
We will use the ideal gas law for this problem:

We know V, which is 7.10
P is 1.11 atm
and T is 31.0 C, or 305 K
R will be 0.08206 L*atm/mol*k, since we are dealing with atmospheres for our pressure.
Now, we just need to solve for n, moles
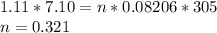
We have 0.321 moles of CO2
Convert to g
The molar mass of CO2 is 44.01 g/mol, so we multiply 44.01 g/mol by 0.321 moles to cancel out the moles and get grams.