Final Answer:
The possible energies of photons emitted from the excited states of the microscopic object are 3 eV, 2 eV, 5 eV, and 10 eV.
Step-by-step explanation:
In this scenario, the emitted photon energies correspond to the differences between the initial energy state (-1 eV, -5 eV, -8 eV, -10 eV, -20 eV) and the excited states. Given that all five states are occupied, we'll consider the transitions between these states.
The transitions occur from higher energy levels to lower ones, generating photons with energies equal to the energy difference between the initial and final states. By calculating these differences, we find that the possible emitted photon energies are 3 eV, 2 eV, 5 eV, and 10 eV.
The energy differences for each transition are:
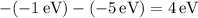
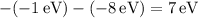
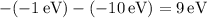

These differences represent the energies of the emitted photons corresponding to the transitions between occupied states. The possible emitted photon energies of 3 eV, 2 eV, 5 eV, and 10 eV reflect the quantized energy transitions within the system, satisfying the conservation of energy as excited states return to lower energy levels.
Understanding the energy transitions between occupied states provides insights into the potential emitted photon energies, illustrating the discrete nature of these transitions within the system.