Answer:
3.Test for unsaturation is carried out by either Baeyer's test or bromine water test.
(a) What is Baeyer's reagent?
Ans:
Baeyer's reagent is an alkaline solution of Potassium Permanganate
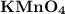 .
.
(b) How does it detect presence of double bond in organic compound? Write the reaction involved.
Ans:
When a dilute alkaline solution of
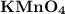 is added to the given unsaturated organic compound, if the purple color of permanganate solution disappears, the presence of double bond is indicated.
is added to the given unsaturated organic compound, if the purple color of permanganate solution disappears, the presence of double bond is indicated.
For example:
2
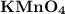 + 2KOH
+ 2KOH

![\bold{2K_2MnO_4 + H_2O+[O]}](https://img.qammunity.org/2024/formulas/chemistry/high-school/6t8qffkv8hiwaeb6t4b2r48bc7gvbe47xt.png)
![\bold{CH_2=CH_2(ethene) +H_2O+[O]\longrightarrow CH_2OH-CH_2OH (ethylene \:glycol :Colourless)}](https://img.qammunity.org/2024/formulas/chemistry/high-school/h09b9gsf8f5u4nk6bjt71c5kjxgpjcq8sr.png)
(c) Write the product when ethyne is treated with Baeyer's reagent.
Ans:
Product will not produce as ethyne doesn't contain double bond.
(d) Benzene (an aromatic hydrocarbon) does not give Baeyer's test though it has double bonds. Why?
Ans:
As Benzene is an unsaturated compound. The structure of benzene contains 3 alternate single double bonds.
(e) Write the reaction for bromine water test for ethyne.
Ans:
Reaction:
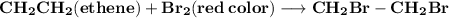
(1,2-dibromoethane (colorless}
Kolbe electrolytic method can be used to prepare alkane, alkene and alkyne.
(a) At which electrode, alkane, alkene or alkyne is obtained?
Ans:
alkane, alkene or alkyne is obtained at anode.
(b) Which electrolyte should be chosen for alkane, alkene or alkyne for the method? Suggest an example of electrolyte for each.
Ans:
alkane: Potassium or sodium salt of Carboxylic acid
alkene: Potassium or sodium salt of dicarboxylic acid
alkyne: Potassium or sodium maleate
(c) If potassium salt of dicarboxylic acid is taken for the method, which hydrocarbon is prepared?
Ans: If potassium salt of dicarboxylic acid is taken for the method, Alkene hydrocarbon is prepared.
(d) Write the detail process for the electrolysis of aqueous potassium maleate.
Ans: Attachment
(e) Suggest a demerit of the method.
Ans:
Demerits:
- Kolbe electrolysis can produce a mixture of different products, including both desired and undesired ones.
- It requires a significant amount of energy to produce the desired products. This can make the process expensive and inefficient.
- It is limited to the electrolysis of alkanes with low molecular weight, and it may not be suitable for the electrolysis of more complex organic molecules.
- This process generates significant amounts of waste and can produce pollutants that can harm the environment.