Answer:
298. 7 K.
Step-by-step explanation:
Hello!
In this case, since equation we use to compute the heat in a cooling or heating process is:
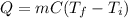
Whereas we are given the heat, mass, specific heat and initial temperature. Thus, we infer that we need to solve for the final temperature just as shown below:
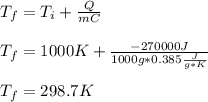
It is important to notice that the iron release heat as water absorbs it, that is why it is taken negative.
Best regards!