Answer:
The new volume of the balloon sample if the total moles inside are increased to 4.5 moles is approximately 3.52 L (3 s.f.).
Step-by-step explanation:
As the temperature and pressure are constant, we can use Avogadro's Law to find the new volume.
Avogadro's Law
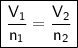
where:
- V₁ is the initial volume.
- n₁ is the initial number of moles.
- V₂ is the final volume.
- n₂ is the final number of moles.
Given 2.3 moles of gas are contained in a balloon with a volume of 1.8 liters, and the final number of moles is increased to 4.5 moles:
- V₁ = 1.8 L
- n₁ = 2.3 mol
- n₂ = 4.5 mol
Substitute the values into the formula and solve for V₂:
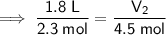
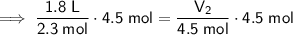
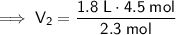
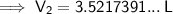
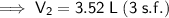
Therefore, the new volume of the balloon sample if the total moles inside are increased to 4.5 moles is approximately 3.52 L (3 s.f.).