Answer:
84.73 mL of 2.46 M Mg(NO₃)₂
Step-by-step explanation:
To calculate the volume of 2.46 M magnesium nitrate [Mg(NO₃)₂] solution needed to make 275 mL of a 0.758 M solution by dilution, we can use the dilution formula.
Dilution formula

where:
- C₁ = Initial concentration
- V₁ = Initial volume
- C₂ = Final concentration
- V₂ = Final volume
In this case, we are diluting the 2.46 M magnesium nitrate [Mg(NO₃)₂] solution to a concentration of 0.758 M, where the final volume is 275 mL, so:
- C₁ = 2.46 M
- C₂ = 0.758 M
- V₂ = 275 mL
Substitute these values into the formula and solve for V₁:

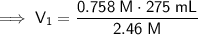
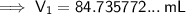
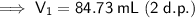
Therefore, we need 84.73 mL of the 2.46 M magnesium nitrate [Mg(NO₃)₂] solution to make 275 mL of a 0.758 M solution by dilution.