Answer:
The final pressure is 0.841 atm (to three significant figures).
Step-by-step explanation:
Since the volume is unchanged, we can use Gay-Lussac's Law to find the pressure of the gas if the temperature increases to 50.00°C.
Gay-Lussac's Law

where:
- P₁ is the initial pressure.
- T₁ is the initial temperature (measured in kelvin).
- P₂ is the final pressure.
- T₂ is the final temperature (measured in kelvin).
Rearrange the equation to solve for P₂:
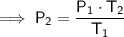
Convert Celsius to kelvin by adding 273.15:
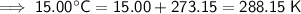
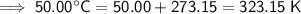
Therefore, the values to substitute into the formula are:
- P₁ = 0.75 atm
- T₁ = 288.15 K
- T₂ = 323.15 K
Substitute the values into the formula and solve for P₂:
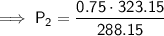
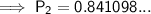
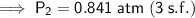
Therefore, the final pressure is 0.841 atm (to three significant figures).