This is a straightforward dilution calculation that can be done using the equation
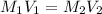
where M₁ and M₂ are the initial and final (or undiluted and diluted) molar concentrations of the solution, respectively, and V₁ and V₂ are the initial and final (or undiluted and diluted) volumes of the solution, respectively.
Here, we have the initial concentration (M₁) and the initial (V₁) and final (V₂) volumes, and we want to find the final concentration (M₂), or the concentration of the solution after dilution. So, we can rearrange our equation to solve for M₂:
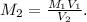
Substituting in our values, we get
![\[M_2=\frac{\left ( 50 \text{ mL} \right )\left ( 0.235 \text{ M} \right )}{\left ( 200.0 \text{ mL} \right )}= 0.05875 \text{ M}\].](https://img.qammunity.org/2022/formulas/chemistry/college/l7286uevb0eeng9euyknh3ab8s5u1h3cnc.png)
So the concentration of the diluted solution is 0.05875 M. You can round that value if necessary according to the appropriate number of sig figs. Note that we don't have to convert our volumes from mL to L since their conversion factors would cancel out anyway; what's important is the ratio of the volumes, which would be the same whether they're presented in milliliters or liters.