Answer:
It's 1.0407 Kilograms of oxygen (O2) in that container
Step-by-step explanation:
according to ideal gas law:

P: pressure, V: volume, n: moles, R: gas constant = 0.0821, T: temperature in Kelvin
Temperature in Kelvin = Temperature in Celsius + 273
Gas constant (R) is changed by changing pressure units (while using atm, R = 0.0821 atm•L/mol•K )
by substituting with given data:
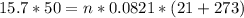
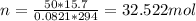
So, O2 mass (Molar mass of O2 = 32 g/mol) = 32.522 * 32 = 1040.709 grams = 1.0407 kilograms