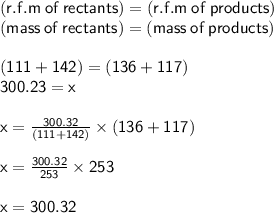Answer:
The mass remains the same since stoichiometrically one mole reacts and one mole is formed
Step-by-step explanation:
Calcium chloride is reacting with Sodium sulphate to form a white precipitate of calcium sulphate.
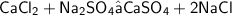
From the equation, 1 mole of calcium chloride forms 1 mole of calcium sulphate.
R.F.M of CaCl2 = 40 + (35.5×2) = 111
R.F.M of CaSO4 = 40 + 32 + (16×4) = 136
R.F.M of Na2SO4 = (23×2) + 32 + (16×4) = 142
R.F.M of 2NaCl = 2[23 + 35.5] = 117