The age of the fossil is 13, 408 years
Half-life is the time required for a substance to decrease or decay by half of its initial quantity.
The half life of carbon-14 is 5730 years
N(t) = 20/100 × N(o)
where N(t) is the found value and N(o) is the intial value.
N(t) = 0.2N(o)
N(t) = N(o) e⁻ˣⁿ
where n is the number of years and x is the decay constant
decay constant = 0.693/half life
decay constant = 0.693/ 5730
= 0.00012
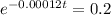
-0.00012t = ln0.2
-0.00012t = -1.609
t = -1.609/-0.00012
t = 13408 ( nearest years)