The number of ions in the compound is obtained as
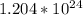 .
.
By acting as a link between the atomic/molecular and macroscopic scales, the mole enables chemists to measure a substance's concentration according to the quantity of particles it contains.
An essential idea in chemistry, the mole is a key component of the International System of Units (SI).
In the compound we can see that there are two
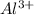 ions and the number of ions in the compound is given by 6.02 *
ions and the number of ions in the compound is given by 6.02 *

We have that;
Number of ions = 2 * 1 * 6.02 *

=
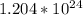
Missing parts;
Consider the compound Al₂
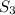 .
.
How many ions of
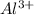 are
are
present in 1.0 mole of Al2S3