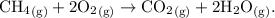The general chemical equation for the complete combustion (or burning) of some hydrocarbon fuel is, in words, as follows:
hydrocarbon fuel and oxygen gas react to produce carbon dioxide gas and water vapor,
or, using chemical notation,
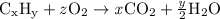
where
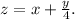
Don't worry if that looks daunting. In our case, we are given the identity of the fuel (methane, or CH₄), so our equations will be straightforward.
In words: Methane gas and oxygen gas react to produce carbon dioxide gas and water vapor (or methane gas + oxygen gas → carbon dioxide gas and water vapor).
In chemical notation: