Final answer:
To find the number of neutrons in the isotope
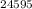 Am, subtract the atomic number (95) from the mass number (245), resulting in 150 neutrons.
Am, subtract the atomic number (95) from the mass number (245), resulting in 150 neutrons.
Step-by-step explanation:
The question asks how many neutrons are in the isotope 24595Am. To find the number of neutrons in an isotope, you subtract the atomic number from the mass number. In this case, the atomic number of Am (Americium) is 95, which is the number of protons, and the mass number is 245.
Number of neutrons = mass number - atomic number = 245 - 95 = 150.
Therefore, the correct answer is C. 150, which represents the number of neutrons in the isotope
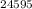 Am.
Am.